Why does atomic radii decrease across a period. Across a period effective nuclear charge increases as electron shielding remains constant.
Why Do Atomic Radi Decrease From Left To Right Across Chegg Com
Why does atomic radius decrease across a period.
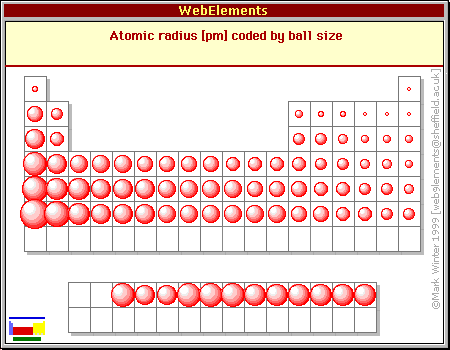
Why does atomic radius decrease across a period. A higher effective nuclear charge causes greater attractions to the electrons pulling the electron cloud closer to the nucleus which results in a smaller atomic radius. Across a period effective nuclear chargeeffective nuclear charge is the net positive charge experienced by an electron in a.

How Does Atomic Radius Change From Left To Right Across A Period In The Periodic Table Socratic
Why Does Atomic Radii Decrease Going From The Bottom Left To The Upper Right Of The Periodic Table Socratic

Trends In The Periodic Table Atomic Radius Atomic Radii Trends And Explanations Atomic Radius Decreases Across A Period Because Each Successive Element Ppt Download
What Is The Trend In Atomic Radius As You Go Across A Period Socratic
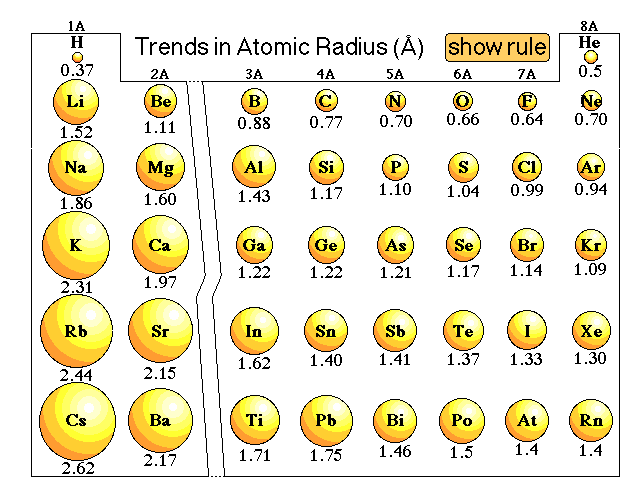
The First Property To Explore Is Atomic Radius
How Does Atomic Size Vary On Moving Across A Period From Left To Right And In A Group Top To Bottom Why Quora
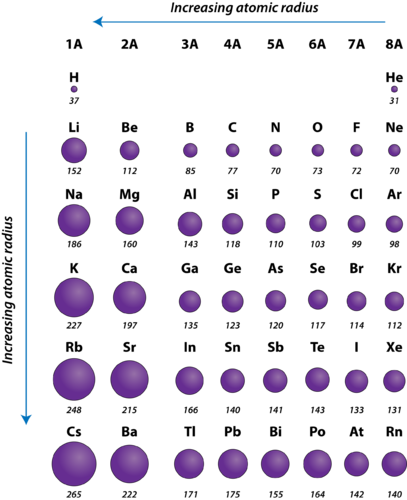
Periodic Trends Atomic Radius Chemistry For Non Majors
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

